FDA approves updated coronavirus shots for young children
< < Go Back
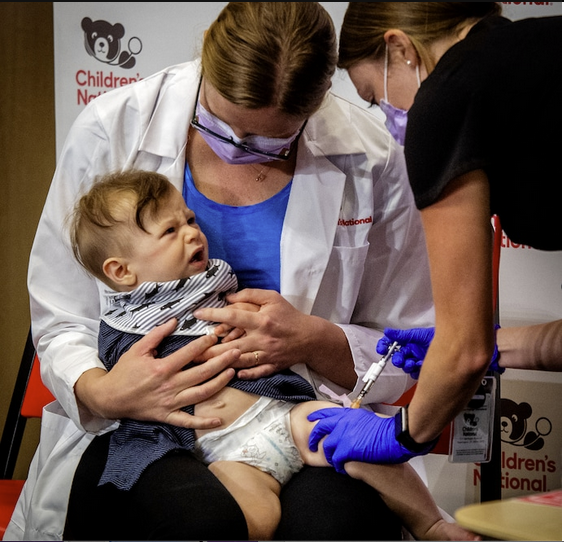
The ‘bivalent’ injections target omicron subvariants and the original virus
Federal regulators Thursday authorized an updated booster shot of Moderna’s coronavirus vaccine for young children, saying the inoculation would offer increased protection amid a wave of respiratory illnesses that is increasing peril for youngsters. This means the youngest Americans will have access to variant-targeting boosters already available to older children and adults.
The Food and Drug Administration, in a statement, said children 6 months through 5 years old would be eligible for the booster — known as a “bivalent” shot targeting the omicron subvariants BA.4 and BA.5 and the original version of the virus — two months after they had completed Moderna’s two-dose primary series.
More From The Washington Post (subscription required):